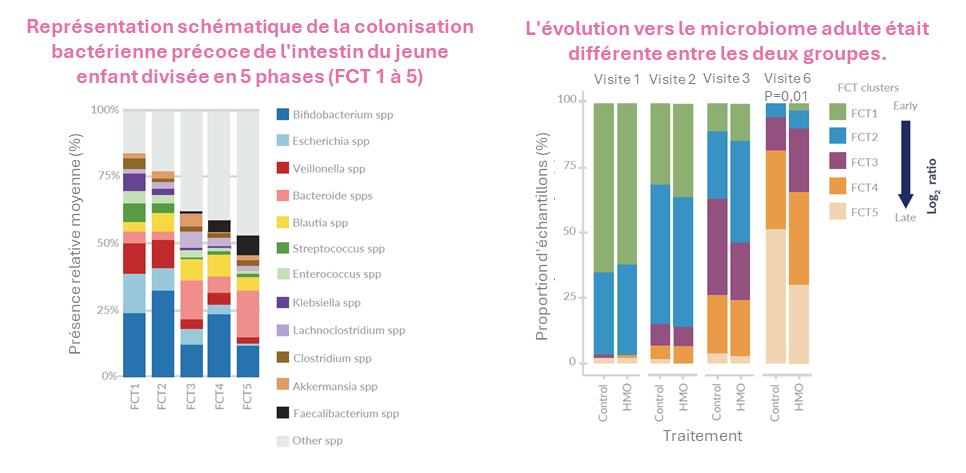
Dans cette étude randomisée multicentrique, les nourrissons (≤12 mois) ont reçu Althéra® HMO (n=97) ou Althéra® (n=97) jusqu'à ce qu'ils atteignent l'âge de 12 mois.
Principaux résultats:
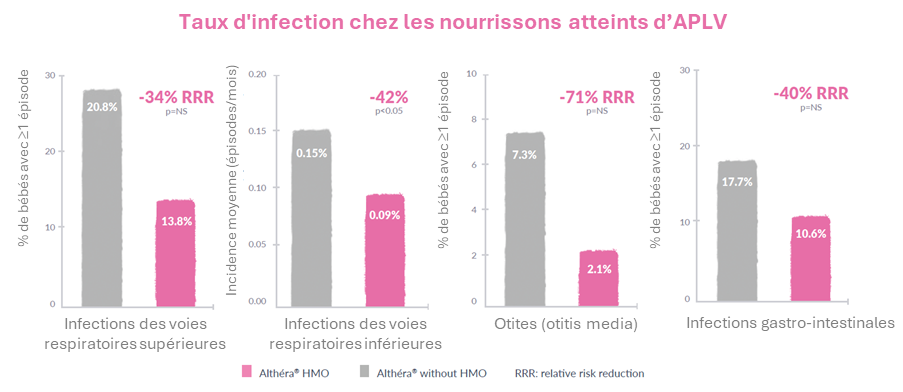
Une croissance normale et une réduction des symptômes de l’APLV ont été constatées dans les deux groupes. En outre, une réduction significative (p<0,05) des infections des voies respiratoires supérieures a été constatée dans le groupe Althéra® HMO. En outre, une réduction du nombre d'infections des voies respiratoires inférieures, d'otites et d'infections gastro-intestinales a également été constatée.
Reference: Vandenplas Y, Żołnowska M, Berni Canani R, Ludman S, Tengelyi Z, Moreno-Álvarez A, Goh AEN, Gosoniu ML, Kirwan BA, Tadi M, Heine RG, Cinnamon Study Investigator Group. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow's Milk Protein Allergy: A Randomized, Multi-Center Trial. Nutrients. 2022 Jan 26;14(3):530. doi: 10.3390/nu14030530.
Vous pouvez lire les grandes lignes de l'étude dans l'infographie ci-dessous.
CINNAMON STUDY: GROWTH, TOLERANCE AND SAFETY OF AN EXTENSIVELY HYDROLYZED FORMULA CONTAINING TWO HUMAN MILK OLIGOSACCHARIDES IN INFANTS WITH COW’S MILK PROTEIN ALLERGY
Vandenplas Y et al. Nutrients. 2022 Jan 26;14(3):530.
Study objectives
To evaluate if an extensively hydrolyzed formula (eHF) supplemented with two human milk oligosaccharides (HMO; 2’-Fucosyllactose [2’-FL] and Lacto-N-neotetraose [LNnT]), with a reduced protein content, supports normal growth and is well tolerated in infants with cow’s milk protein allergy (CMPA).
Subjects and methods
Multicenter, randomized study included infants with CMPA who received a whey-based eHF with 1.0 g/L 2’-FL and 0.5 g/L LNnT (Althéra® HMO) (n=97) or a commercially available whey-based eHF without HMO (Althera®) (n=97), as control formula. Infants were followed to 12 months of age.
Endpoints
Daily weight gain from baseline (V0) to 4 months (V4) and 12 months (V6) (primary endpoint). Weight-for-age, lengthfor- age and head circumference-for-age Z-scores, clinical tolerance and efficacy (Cow’s Milk-related Symptom Scores; CoMiSS®), rates of adverse events and medication use until 12 months of age (secondary endpoints).
Results
- Non-inferior daily weight gain was shown for Althéra® HMO from V0 to V4 vs. Althera® without HMO (19.38g/d vs. 20.12g/d; p=0.0049)
- There were no significant group differences for any of the anthropometric parameters at any study time point
- Althéra® HMO was well tolerated and mean CoMiSS® scores were significantly reduced from 12.1 to 3.4 (p<0.001; V0 to V1)
- Number of adverse events was similar between groups
- The frequency of upper respiratory tract infections was significantly reduced (p<0.05), while a trend toward a reduction in the rate of respiratory tract infections, otitis media, gastrointestinal infections as well as the use of antibiotics and antipyretics was reported in those consuming Althéra® HMO
- A significant 100% reduction was observed for otitis media in the per protocol group (p<0.05) consuming Althéra® HMO
Conclusion
Infants with CMPA fed Althéra® HMO, with a reduced protein content, achieved symptom relief and normal growth. A reduction of respiratory tract, ear and gastrointestinal infections was observed, as well as a lower use of associated medication.